What is Chemiluminescence...
Chemiluminescence (CL) is defined as the production of electromagnetic radiation (ultraviolet, visible or infrared) observed when a chemical reaction yields an electronically excited intermediate or product, which either luminesces (direct CL) or donates its energy to another molecule responsible for the emission (indirect or sensitized CL).
When a Nitrogen containing sample is combusted at 1000 ℃, Nitrogen Oxide (NO) is formed: R-N + O2 –> NO + H2O + CO2
After conditioning of the combusted sample, the formed NO is led into a reaction chamber, where electronically generated Ozone is added which reacts with the Nitric Oxide which forms Nitrogen Dioxide in an excited state (NO2*). The excited NO2 emits light as it reverts to a lower level energy state. The emitted light is detected by a Photomultiplier Tube (PMT).
The amount of the emitted light which is detected, corresponds with the amount of NO. This represents the amount of Total Nitrogen present in the sample and is so-called the reduced pressure Chemiluminescence detection technique. Surplus of Ozone is converted to oxygen by a heated tube to avoid this will lead to the environment.
The equations for these reactions are:
NO + O3 –> NO2*
NO2* –> NO2 + hv
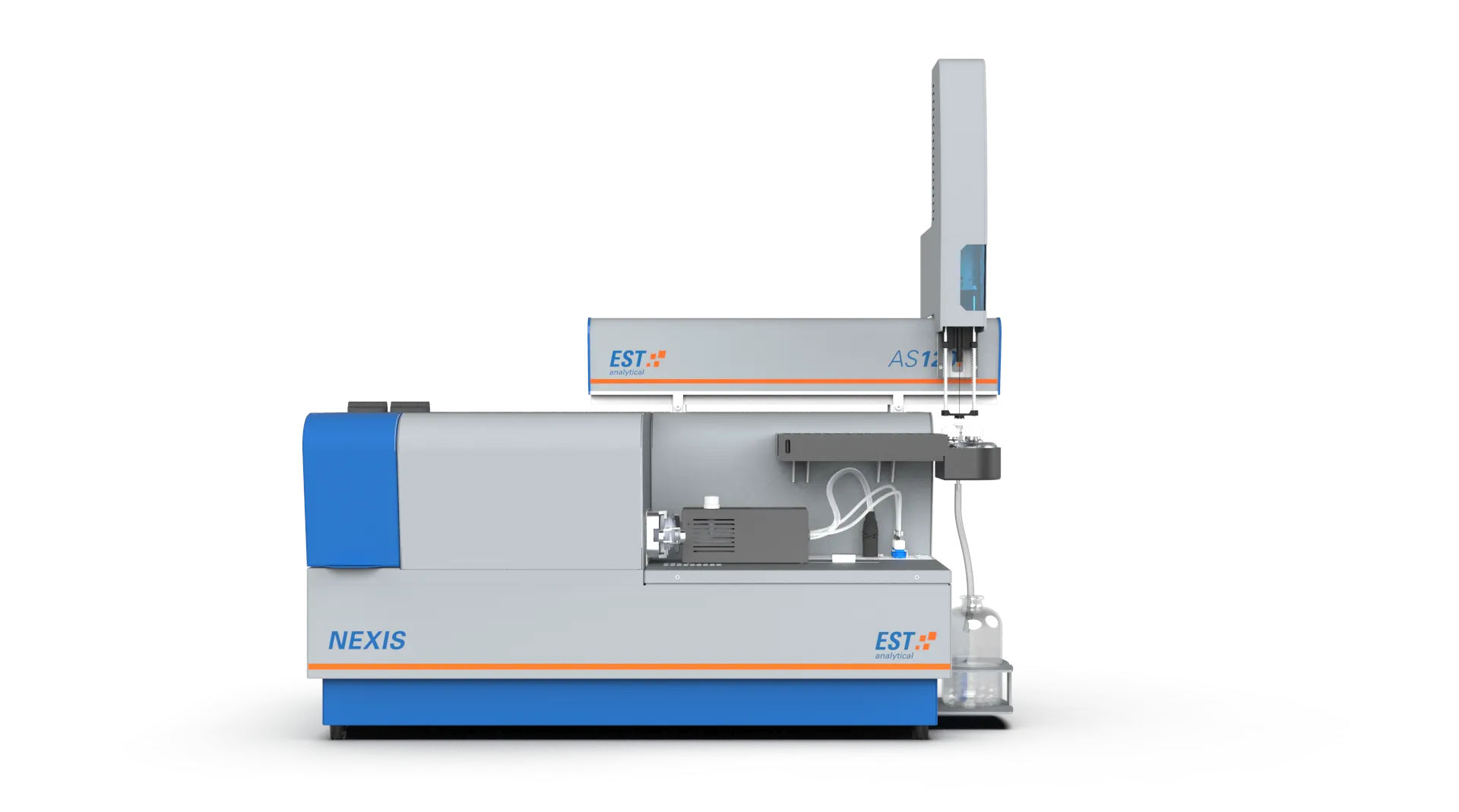
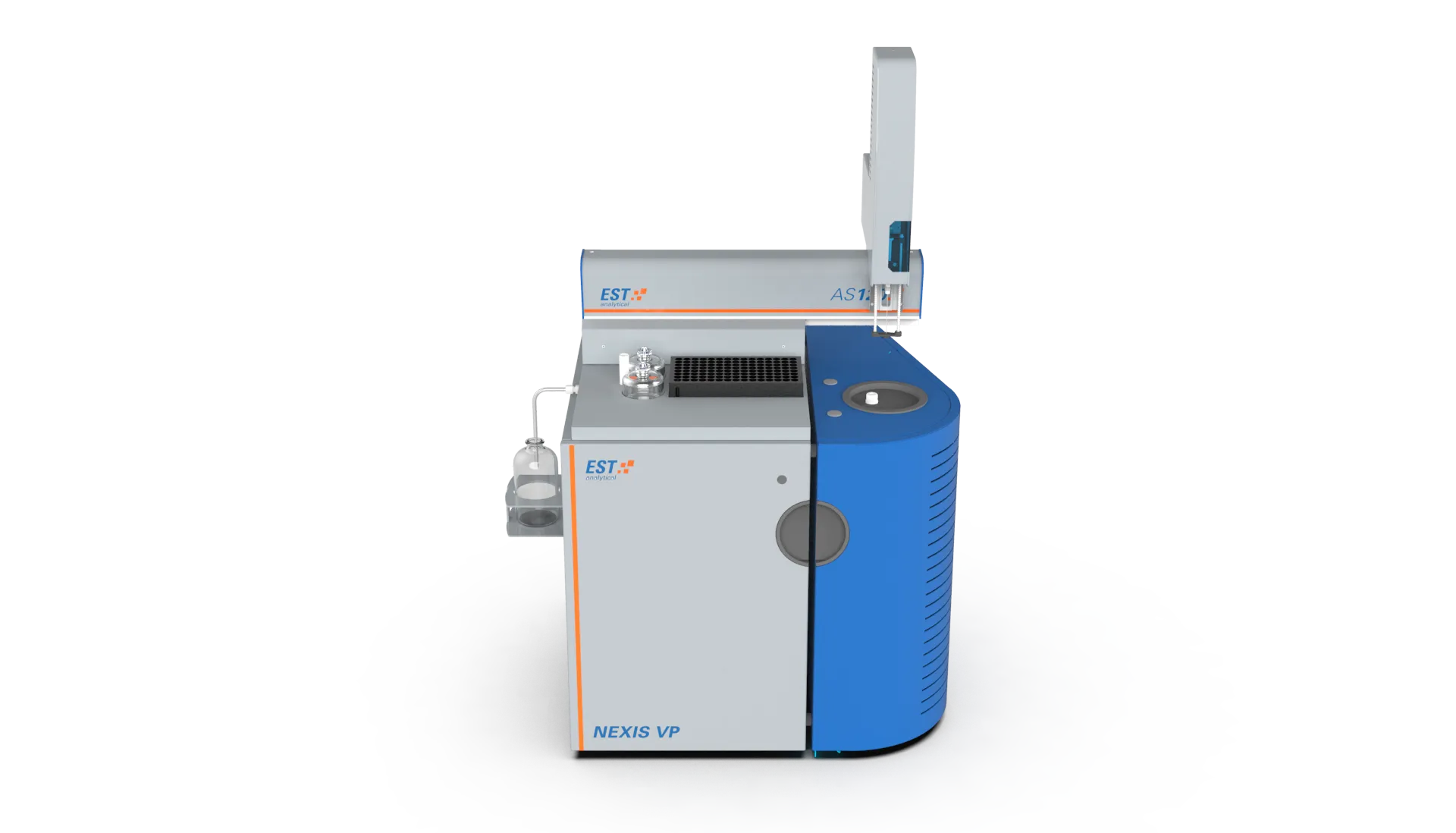
Elemental combustion analyzers that make use of this detection technique: NEXIS TN, NEXIS VP TN
International methods carried out by using this technique: ASTM D4629, ASTM D5762, ASTM D6069, ASTM D7184, SHT 0657